- Press Office
- Press releases 2020
- Cellular powerplant recycles waste gases
Cellular powerplant recycles waste gases
Waste gases of many branches of industry – for example in steel mills – contain mainly carbon monoxide and carbon dioxide. Nowadays, these harmful and greenhouse gases are simply blown into our atmosphere, but this may soon change. The idea is to use the power of bacteria to turn toxic waste gases into valuable compounds such as acetate or ethanol. These can be used afterwards as biofuels or basic compounds for synthetic materials. The first real-size test plants are already under evaluation, using this conversion at an industrial scale, and the stars of these process are bacteria that devour carbon monoxide, carbon dioxide and dihydrogen, among which Clostridium autoethanogenum is by far the favorite.
“In this microbe, the main lines of the metabolism used to operate the gas conversion have been characterized,” says Tristan Wagner, leader of the group Microbial Metabolism at the Max Planck Institute for Marine Microbiology. “But there are still a lot of question marks at the molecular level”. The one in focus of the scientists from Bremen: How is the toxic carbon monoxide processed by enzymes at such stunning efficiency?
Big surprise in a crystal
The molecular-level knowledge of the carbon monoxide conversion is derived from studies performed in the species Moorella thermoacetica. This is a convenient and well-studied marine model organism but exhibits a poor ability to detoxify waste gases, unlike Clostridium autoethanogenum. Both bacteria use the same enzyme to convert carbon monoxide: the CO-dehydrogenase/Acetyl-CoA synthase, shortened as CODH/ACS. It is a very common enzyme which existed already in primeval times of the earth. “Since both species use the same enzyme to convert carbon monoxide, we were expecting to see exactly the same structure with eventually minor differences,” says Wagner.
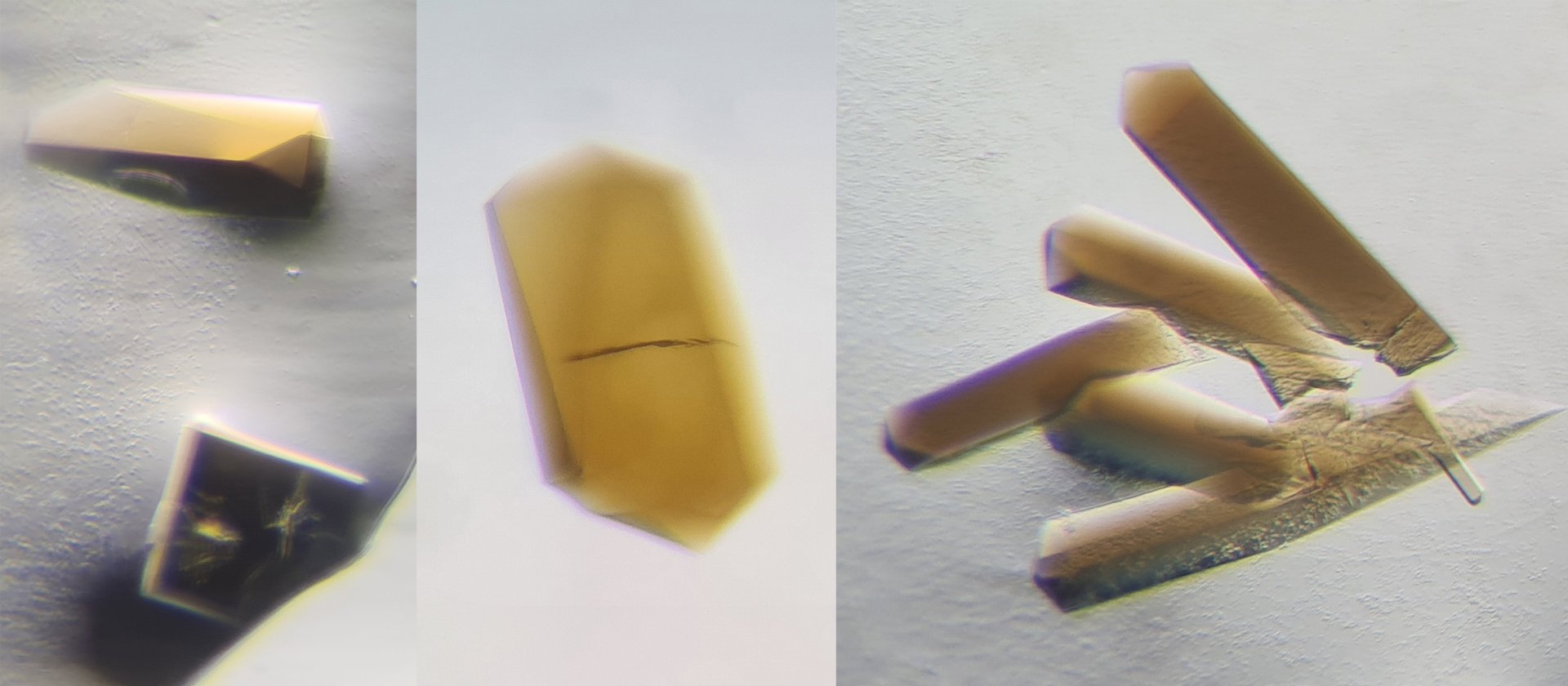
For their research, Wagner and his colleague Olivier N. Lemaire are studying the bacterium Clostridium autoethanogenum to understand how it can thrive at the thermodynamics of Life, using a metabolism similar to that of the first living forms. Olivier N. Lemaire grew the bacteria and purified its CODH/ACS in absence of oxygen, which is detrimental to the enzyme. The two scientists used the crystallization method to obtain crystals of the enzyme CODH/ACS and determine the protein 3D-structure by X-ray crystallography. “When we saw the results, we couldn’t believe our eyes,” says Wagner. “The CODH-ACS interface from Clostridium autoethanogenum drastically differs from the model of Moorella thermoacetica, even though it was the same enzyme and similar bacteria”.
Same ingredients, different architecture
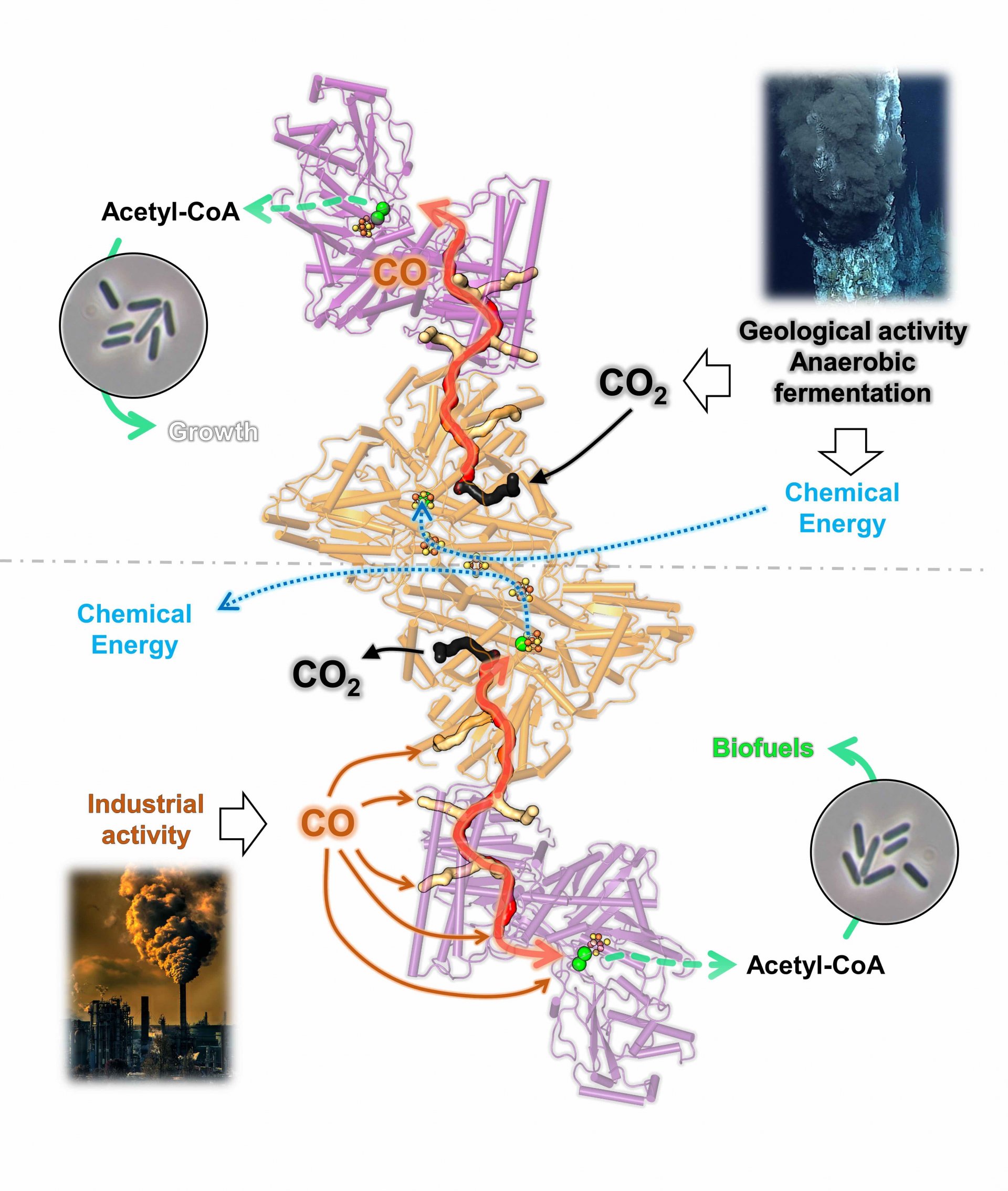
Afterwards, the two researchers carried out further experiments to prove that the first structure was not an artifact but the biological reality. Following experiments confirmed the initial model. Thus, the discovery clearly proves wrong the previous assumption that the enzyme CODH/ACS always has the same overall structure. “The enzyme of Moorella thermoacetica has a linear shape,” explains Olivier N. Lemaire, first author of the study, which was recently published in the scientific journal BBA Bioenergetics. “In Moorella thermoacetica, the enzyme produces carbon monoxide in the CODH and uses in the ACS. Between them, it is trapped and funneled through a sealed gas-channel. ACS will ultimately synthesize acetyl-CoA, a building block further processed into acetate and ethanol. The rest of the cell do not see any carbon monoxide”.
But Clostridium autoethanogenum absorbs carbon monoxide directly. “In Clostridium autoethanogenum the enzyme CODH/ACS has not only one opening, but several. In this way it can collect as much carbon monoxide as possible and conduct it into a whole system of tunnels, operating in both directions”, says Lemaire. “These results show a reshuffling of internal gas-tunnels during evolution of these bacteria, putatively leading to a bidirectional complex that ensures a high flux of carbon monoxide conversion toward energy conservation and assimilation of carbon monoxide, acting as the main cellular powerplant”. At the end of the process also acetate and ethanol are generated, which can be used to produce fuels.
“We now have a picture of what this very efficient and robust enzyme looks like”, says Tristan Wagner. “But our discovery is only one step further. Among other things, it is still an open question how the bacterium can survive and use carbon monoxide to feed their whole cellular energy needs. We have some hypotheses, but we are still at the beginning. To understand the whole chemical process of converting carbon monoxide to acetate and ethanol, further proteins need to be studied”.
Original publication
Olivier N. Lemaire and Tristan Wagner: Gas channel rerouting in a primordial enzyme: Structural insights of the carbon-monoxide dehydrogenase/acetyl-CoA synthase complex from the acetogen Clostridium autoethanogenum. BBA – Bioenergetics, 2020
DOI: 10.1016/j.bbabio.2020.148330
Please direct your queries to:
Head of Group
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany