Page path:
- Departments
- Department of Biogeochemistry
- Biogeochemistry Group
- Projects
- Nutrient Cycling in Response to Global Change
Nutrient Cycling in Response to Global Change
In the past few decades, a decline in dissolved oxygen has been observed in global oceans, resulting in expanding oceanic OMZs and increasing coastal hypoxia that are likely exacerbated by the projected ocean warming and other anthroporgenic pressures. Meanwhile, rising atmospheric CO2 levels have caused ocean acidification worldwide, which would also continue in the future. These phenomena would likely have important implications for nutrient cycling in the oceans.
Response to Expanding OMZs
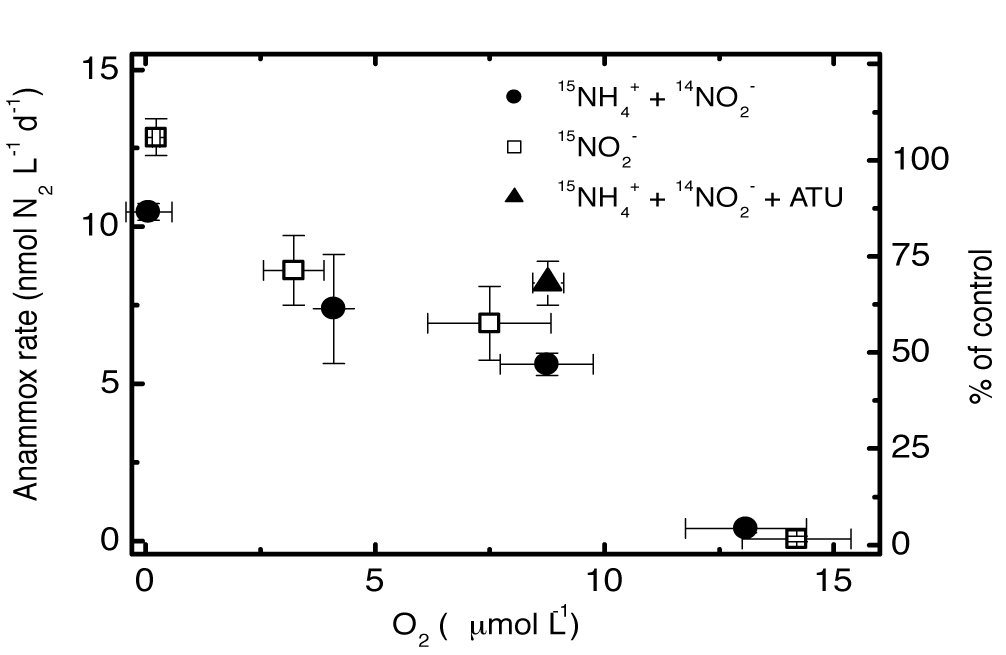
The global expansion of OMZs implies that a larger ocean volume would be subject to oceanic N-loss. In collaboration with the IFM-GEOMAR (Kiel), the effects of oxygen concentrations on nutrient regeneration and N-loss processes were analysed in manipulative experiments with varying O2 concentrations. Anammox remained active up to ~15 µM of O2 in the Namibian and Peruvian OMZs. Furthermore, our experimental results indicated that NO3- reduction in the OMZs could tolerate even up to ~20 µM of O2. Meanwhile, nitrifying activities (including both ammonium and nitrite oxidation) may continue at apparent anoxic conditions (<1µM O2) given sufficient substrate. The respective O2-tolerance of these processes enabled their spatial coexistence and thus a tightly coupled N-cycle in the OMZs. The results arising from this project will enhance our understanding of the impact of low-oxygen conditions and potential future changes on the nutrient inventory of the tropical oceans.
Is sulfide in OMZ waters an increasing phenomenon as a result of global change?
Hydrogen sulfide is a toxin that is more deadly to multi-cellular life than cyanide. It is abundantly present in anoxic marine sediments and the water column of some isolated basins, where it is formed upon sulfate respiration by sulfate reducing bacteria. In open waters, on the other hand, substantial concentrations of H2S are generally believed to be extremely rare and large expanses of sulfide-containing waters have been so far reported only for the Namibian and Indian shelfves (e.g. Lavik et al., 2009). It is possible that the widespread occurrence of hydrogen sulphide is a relatively recent phenomenon resulting from an intensification of oxygen deficiency in this area, as in other parts of the coastal ocean, due to human activities. Given the economical importance of fisheries in the coastal waters, a deeper understanding of these toxic sulfidic events is urgently needed. We are, therefore, studying the factors regulating the inititation and termination of these events. Results from Namibia indicate that H2S was oxidized anaerobically with NOx by distinctive blooming bacteria (Lavik et al., 2009).
Tip of the iceberg? A large patch of discolored surface waters off Namibia attributed to toxic hydrogen sulphide release from the seafloor. Many of these naturally occurring events may go unnoticed by satellite because bacteria consume sulphide before it reaches the surface.
Photo credit: Jacques Descloitres, MODIS Rapid Response Team, NASA/GSFC, visibleearth.nasa.gov/view_rec.php?id=4976
Effects of Ocean Acidification on Nutrient Cycling in Marine Sediments
Atmospheric carbon dioxide (CO2) has risen 31% since pre-industrial times, with 30% being absorbed by the surface ocean, resulting in a pH reduction of ~0.1 in surface seawater. If CO2 emissions continue to rise in an IS92a “business as usual” scenario, models predict a further decrease of 0.3-0.5 in pH by 2100. Thus far, little is known about the potential effect of ocean acidification on the nutrient biogeochemistry in coastal sediments. Using a mesocosm approach, variations in nutrient cycling within coastal sediments are investigated. Sediments kept in flow through reactors are fed with seawater maintained at desginated pCO2 levels (170, 380, 750 and 1200 ppm). Initial results show a negative correlation between nitrate concentrations in the seawater reservoirs and pCO2 indicating decreasing nitrification with decreasing pH
Experimental Design in theory and in application (courtesy Hannah Marchant).