Page path:
- Departments
- Department of Biogeochemistry
- Biogeochemistry Group
- People
- Research Projects
Research Projects
The impact of biogeochemical sulfur cycling on the oxygen isotope composition of sulfate
Many aspects of the processes influencing the oxygen isotope composition of sulfate are not fully understood:
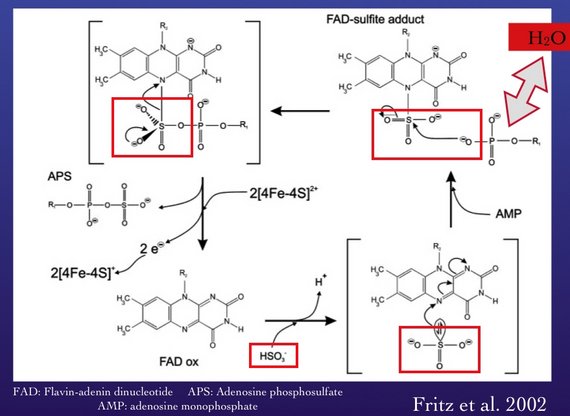
• We know that microbial sulfate reduction causes changes in the oxygen isotope composition of residual sulfate, and that the changes depend on the oxygen isotope composition of water. However, we do not know at what intermediate stage in sulfate reduction this exchange occurs. Candidates are APS or sulfite and a new mechanism has been postulated (Wortmann et al., 2007). This mechanism involves the transfer of oxygen from AMP to sulfite in the reversible reaction from sulfite to APS (Fritz et al. 2002). A consequence of this mechanism is that kinetic oxygen isotope fractionation could become important under certain conditions.
• We know that microbial sulfate reduction causes changes in the oxygen isotope composition of residual sulfate, and that the changes depend on the oxygen isotope composition of water. However, we do not know at what intermediate stage in sulfate reduction this exchange occurs. Candidates are APS or sulfite and a new mechanism has been postulated (Wortmann et al., 2007). This mechanism involves the transfer of oxygen from AMP to sulfite in the reversible reaction from sulfite to APS (Fritz et al. 2002). A consequence of this mechanism is that kinetic oxygen isotope fractionation could become important under certain conditions.
• The oxygen isotope composition of sulfate that is produced by disproportionation of elemental sulfur seems to depend on the type of sulfide scavenger, such as manganese oxide or Fe(III) and Fe(II) compounds (Böttcher and Thamdrup, 2001). This is somewhat surprising, since these sulfide scavengers are not directly involved in the disproportionation, and it may indicate that the effectiveness of the sulfide scavengers affects the speed of the oxidation of sulfur intermediates that exchange oxygen with water.
• The evolution of the oxygen isotope composition of seawater sulfate is loosely tied to the one of seawater by oxidative processes. Therefore, trends in the oxygen isotope composition of seawater should leave an imprint in the one of seawater sulfate. However, apparently, there is no correlation at all, and this paradox needs to be resolved.
• The proxies used for reconstructing the history of the oxygen isotope composition of seawater sulfate are marine barite and carbonate associated sulfate (CAS). However, these proxies may not be reliable: obviously, there is an oxygen isotope fractionation related to the formation of barite (Turchyn and Schrag, 2006), and the reliability of CAS as carrier of the oxygen isotope composition of seawater sulfate has been challenged.
• The evolution of the oxygen isotope composition of seawater sulfate is loosely tied to the one of seawater by oxidative processes. Therefore, trends in the oxygen isotope composition of seawater should leave an imprint in the one of seawater sulfate. However, apparently, there is no correlation at all, and this paradox needs to be resolved.
• The proxies used for reconstructing the history of the oxygen isotope composition of seawater sulfate are marine barite and carbonate associated sulfate (CAS). However, these proxies may not be reliable: obviously, there is an oxygen isotope fractionation related to the formation of barite (Turchyn and Schrag, 2006), and the reliability of CAS as carrier of the oxygen isotope composition of seawater sulfate has been challenged.
Sulfur species other than sulfide and sulfate (i.e. elemental sulfur and sulfite) in biochemical sulfur cycling
There are two aspects related to intermediate sulfur species that intrigue me:
• Sulfur disproportionation is generally accepted as being of major importance for microbial sulfur cycling. However, it remains unclear to me how the elemental sulfur required for this cycling is formed, i.e. if it is really derived from an abiotic oxidation of sulfide to elemental sulfur.
• Sulfur disproportionation is generally accepted as being of major importance for microbial sulfur cycling. However, it remains unclear to me how the elemental sulfur required for this cycling is formed, i.e. if it is really derived from an abiotic oxidation of sulfide to elemental sulfur.
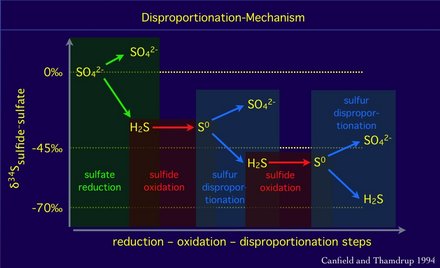
Sulfur isotope fractionation and disproprtionation
The sulfur isotope fractionation by disproportionation of elemental sulfur can increase the difference between the isotope composition of sulfate and sulfide.
The sulfur isotope fractionation by disproportionation of elemental sulfur can increase the difference between the isotope composition of sulfate and sulfide.
• Assimilatory and dissimilatory sulfate reduction are assumed to be very ancient biological pathways (Shen and Buick, 2003). However, more reduced sulfur species (i.e. sulfides, elemental sulfur) are much more likely to have existed at the origin of life. Thus, I would expect that pathways other than sulfate reduction were first to evolve. Maybe, the stepwise process “sulfate reduction” is made of more ancient building blocks. In this perspective, interpreting the sulfur isotope pattern from the rock record (i.e. the rule of thumb that small isotope fractionations occur when sulfate is limiting) may be misleading.
Interaction of the microbial sulfur cycling with other geochemical cycles
Both biologically induced formation and bioleaching of minerals are important processes in sulfur cycling:
• There is a strong, but yet not understood link between sulfidic environments, pyrite formation and sequestration of molybdenum, whose isotopes are used to reconstruct paleo-redox conditions (Barling et al., 2001, Arnold et al., 2004).
• While bioleaching of sulfides (i.e. pyrite) has been investigated in great detail, basic information about leaching of sulfate minerals is missing. Both leaching of barite and gypsum by sulfate reducers have been observed, and possibly, sulfate associated with carbonates can also be attacked. However, neither potential isotope effects nor leaching mechanisms have been studied.
• There is a strong, but yet not understood link between sulfidic environments, pyrite formation and sequestration of molybdenum, whose isotopes are used to reconstruct paleo-redox conditions (Barling et al., 2001, Arnold et al., 2004).
• While bioleaching of sulfides (i.e. pyrite) has been investigated in great detail, basic information about leaching of sulfate minerals is missing. Both leaching of barite and gypsum by sulfate reducers have been observed, and possibly, sulfate associated with carbonates can also be attacked. However, neither potential isotope effects nor leaching mechanisms have been studied.
The oceanic-atmospheric dimethylsulfide (DMS) cycle
The flux of DMS from the ocean to the atmosphere and its subsequent oxidation is of major importance, since it links atmospheric processes with primary productivity and could have an impact on climate. The current knowledge about the DMS cycle is very limited, i.e. the involved fluxes are hard to estimate.
Sulfur cycling in extreme environments and their link to Mars
The finding of sulfate minerals on Mars, that are likely to have been formed by the evaporation of acid brines, is very stimulating, since it links two extreme environments, saline brines and acid mine drainage. The investigation of such environments can provide us with exciting insights into unknown microbial and geochemical processes. Detection of patterns that indicate presence or absence of life in geologic samples (i.e. three dimensional distribution of minerals, elements and their isotope composition) are essential for the search of extraterrestrial life and the origin of life on our planet.

Presence or absence of life?
Answering this question for a far distant Galaxy seems to be impossibe - but even for our own planet, we have a hard time reading the history of life from the geologic rock record.
Cathode luminescence image of an iron ooide (2mm diameter): Biologically induced or abiogenic in origin?
Answering this question for a far distant Galaxy seems to be impossibe - but even for our own planet, we have a hard time reading the history of life from the geologic rock record.
Cathode luminescence image of an iron ooide (2mm diameter): Biologically induced or abiogenic in origin?


Hunting for pink halite minerals in the brine pools of Searles Lake (Trona, CA). The pink color of the salt crystals is caused by remains of microbes that thrive in the brine.