- Research & Instruments
- How we study - Our instruments and methods
- Instruments and Methods
- Methods
- Analysis of microbial metabolism
Analysis of microbial metabolism
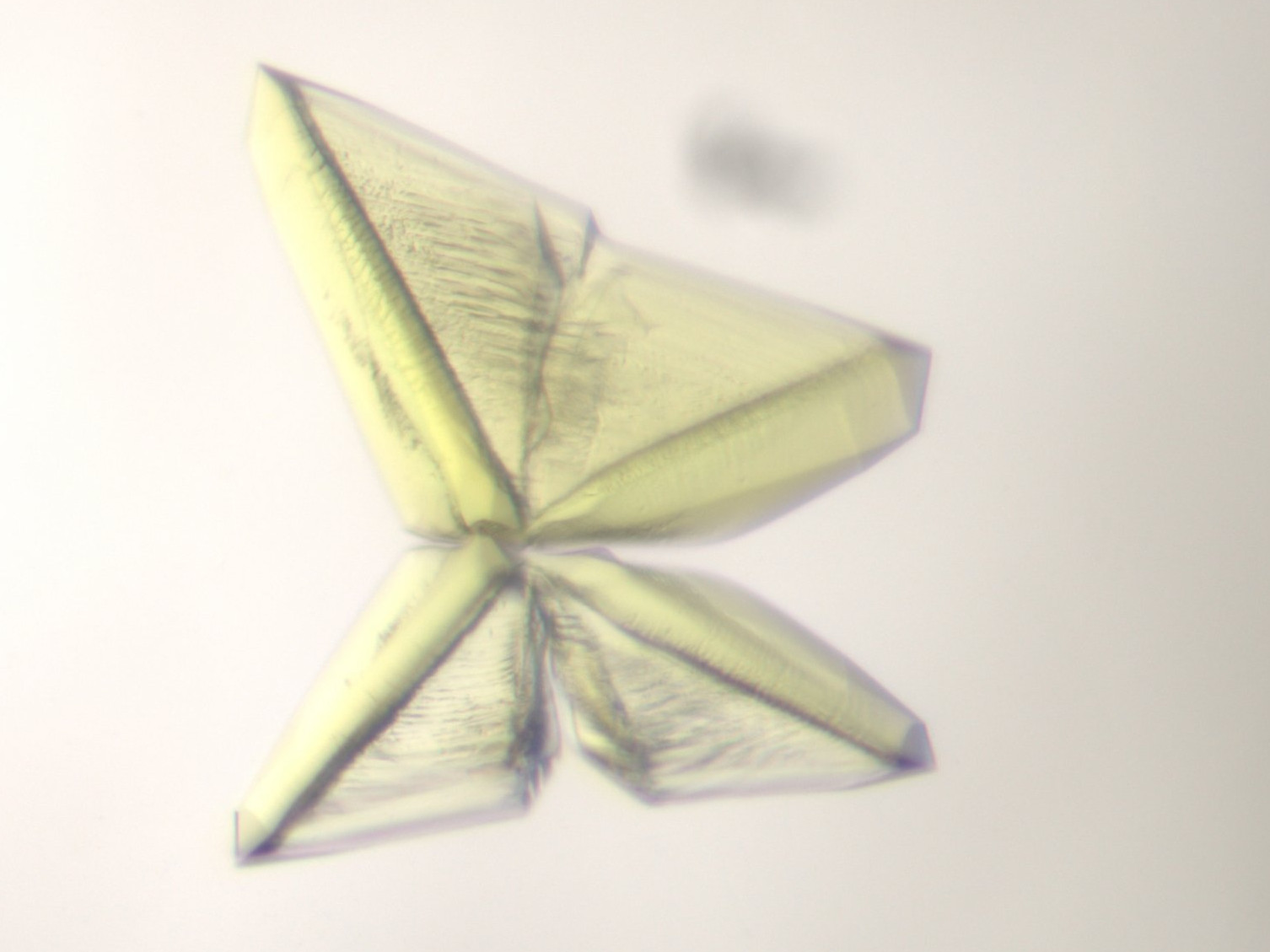
Why is relevant to study microbial metabolism?
The Microbial Metabolism Group aims to understand, at the molecular level, how methanogens are surviving and growing in extreme environments. How do they generate methane from different sources of carbon so efficiently? How do they convert minerals into the elementary bricks of life? And how do they protect themselves against stresses from their natural environment?
How does the analysis of microbial metabolism work?
To find an answer to these questions, we must cultivate these microorganisms and study the different chemical reactions occurring inside them. The enzymes involved in the conversion of minerals and gases are proteins that orchestrate strange reactions highly challenging for chemists. We have to extract these enzymes, and sort them out from other proteins by using the native purification. We then pierce the molecular secret of their reaction by looking at them with X-ray crystallography, which means that we have to crystallize the enzymes, and use X-ray to get their pictures.
The most critical part of his groups’ study is methanogen cultivation: we need special gases, equipments and most importantly the knowledge to take care of them.
Which instruments are important for the analysis?
Autoclave
Autoclaves are used to sterilize solutions and materials, or even waste prior to disposal. You might have heard of autoclaves from the medical sector, for instance to sterilize surgical tools. At the institute we have a hospital-size autoclave, which allows us to even sterilize large devices (fermenter).
Manual Gas-exchanger
Many of our organisms grow anaerobically, so without any oxygen. Therefore, we require the manual Gas-exchanger to make solutions and cultures anaerobic, using the gases nitrogen or a mixture of hydrogen and carbon dioxide.
Automatic Gas-exchanger
This device runs automatically and also removes oxygen from solutions. In this case, the oxygen can only be replaced by nitrogen gas.
Incubator
Our lab has special incubators, which can maintain temperatures up to 80 °C and shake up to 220 rotation per minute. These incubators allow us to provide the perfect growth conditions for many microorganisms cultivated in erlenmeyer or pressure-resistant bottles.
Fermenter
Often, small and pressure protected bottles are used to grow anaerobic microorganisms. The use of fermenter allows us to increase the volume of culture – up to 10 liter and provides a constant amount of gas(es) to stimulate the growth of microorganisms.

Sonication
To extract the proteins within the microorganisms the cells need to be broken. We often break the cells via ultra-sonication. Since this device is rather small, one Sonicator is placed inside an anaerobic chamber (see below).
French Press
The French Press is another way to break the cells but with high pressure, which can be manually adjusted.
Aekta Avant
The Aekta Avant is used to aerobically purify proteins (liquid chromatography) – so in presence of oxygen. However, Aekta systems are also placed inside anaerobic chambers to guarantee a protein purification without oxygen.
Anaerobic chambers
The MicroMet team has five anaerobic chambers.
- The first tent has a nitrogen/carbon-dioxide atmosphere. Anaerobic organisms are “harvested” in this tent and cells can be broken anaerobically by a sonication device.
- Three tents with a hydrogen/nitrogen atmosphere stand in a room which can be kept under 18 °C and yellow-light conditions. This is important as many proteins are light sensitive and could be damaged by normal room light. Two tents comprise all devices required to perform anaerobic protein purifications. Here, we can fractionate and separate proteins from each other. One tent is solely used for anaerobic protein-crystallization. Crystallization is a delicate process, for which reason the tent stands on a anti-vibration table and the atmosphere is kept dust-free via a filter system.
- The last tent has a nitrogen atmosphere and is used for anaerobic robotic crystallization and protein characterization.







Gas chromatographs
The gas chromatographs are used to measure gases and to measure their concentrations, for example, during the growth of cell cultures. Among others, the following gases can be measured: Methane, carbon monoxide, carbon dioxide, hydrogen and oxygen.
Photometer
The photometer measures enzyme activities and records spectra (wavelength scan from 200-1100 nm). A maximum of 18 cuvettes can be measured simultaneously, the smallest sample volume is 5 - 1000 µl. The temperature can be set up to 90 degree celsius.
Research Examples
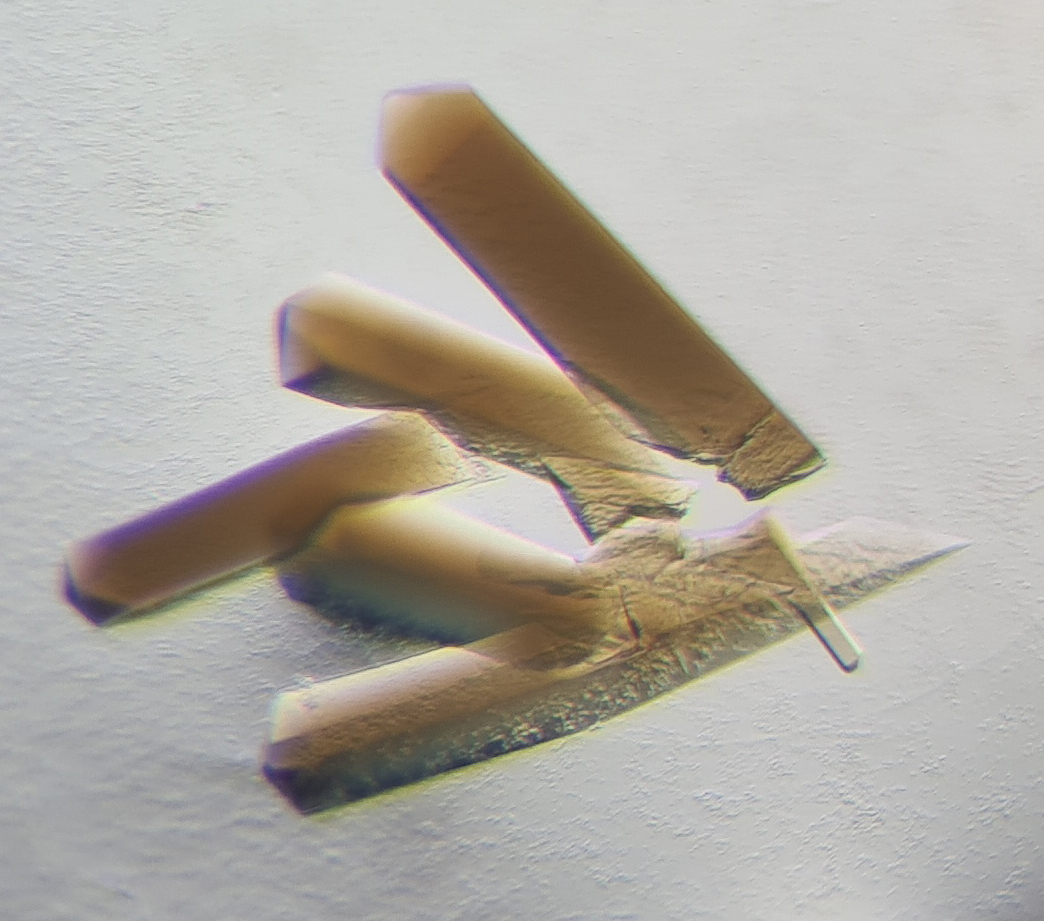
Cellular powerplant recycles waste gases
Carbon monoxide is a very poisonous gas. Humans die within minutes when they inhale it. However, some microorganisms tolerate carbon monoxide and even use it to breathe and replicate. Knowledge about how these bacteria survive opens a window into the primeval times of the earth and the origin of life. At the same time, they might be very useful for the future as they can be used to clean waste gases and produce biofuels.
In this context, scientists from the Max Planck Institute for Marine Microbiology in Bremen have made a surprising discovery. The two scientists used the crystallization method to obtain crystals of the enzyme CODH/ACS and determine the protein 3D-structure by X-ray crystallography. The surprising discovery: The CODH-ACS interface from one bacteria drastically differs from another, even though it was the same enzyme and similar bacteria.
Read more in the press release "Cellular powerplant recycles waste gases"
Original publication
Olivier N. Lemaire and Tristan Wagner: Gas channel rerouting in a primordial enzyme: Structural insights of the carbon-monoxide dehydrogenase/acetyl-CoA synthase complex from the acetogen Clostridium autoethanogenum. BBA – Bioenergetics, 2020
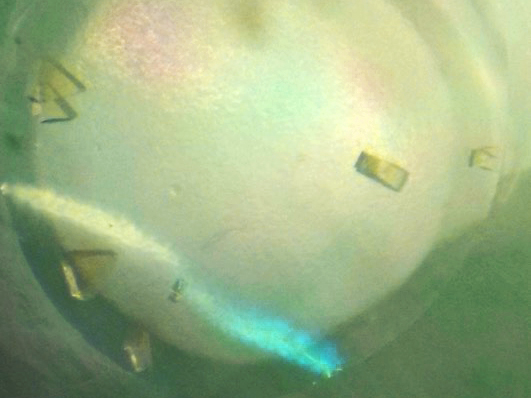
Water at the end of the tunnel
We humans need oxygen to breath – for a lot of microbes it is a lethal poison. That is why microorganisms have developed ways to render oxygen molecules harmless. Microbiologists from Bremen, Marburg and Grenoble have now succeeded in decrypting such a mechanism. They show, how methane-generating microbes transform oxygen into water without causing any damage to the cell. These findings are relevant for future bio-inspired processes.
Read more in the press release "Water at the end of the tunnel"
Original publication
Sylvain Engilberge#, Tristan Wagner#, Philippe Carpentier, Eric Girard, Seigo Shima: Krypton-derivatization highlights O2-channeling in a four-electron reducing oxidase. Chemical Communication, September 2020
DOI: 10.1039/d0cc04557h
# both authors contributed equally to this work
Contact
Head of Group
MPI for Marine Microbiology
Celsiusstr. 1
D-28359 Bremen
Germany









