- Abteilungen
- HGF MPG Brückengruppe für Tiefsee-Ökologie und -Technologie
- Forschungsthemen
Forschungsthemen
The Joint Research Group on Deep-Sea Ecology and Technology, headed by Prof. Antje Boetius, is co-funded by the Alfred Wegener Institute Helmholtz Center for Polar and Marine Research and the Max-Planck-Institute for Marine Microbiology. We combine the expertise of the MPI branch (former Microbial Habitat Group, 2003-2010) in marine microbial ecology and biogeochemistry, the development of new molecular, biological and in situ analytical methods, with the expertise of the AWI branch (former Deep-Sea Research group, 1999-2008) in studying polar environments, pursuing long-term environmental observations, and sampling in the deep sea.
The main goals of the HGF-MPG Deep-Sea Ecology and Technology group are (1) to obtain “true” quantitative insight to ecosystem structure, dynamics and biogeochemical fluxes based on in situ measurements (2) to show the effects of global change on deep-sea ecosystems and including variations in microbial to macrofaunal biodiversity on relevant spatial and temporal scales, and (3) to unravel the functioning of key microorganisms in specific deep-sea environment. To quantify transport and reactions in submarine and subglacial deep-sea ecosystems we develop and improve in situ instruments and describe habitats with a variety of geochemical methods. To study the inhabiting (micro)fauna and to describe their functions we use state of the art molecular approaches.
Here we report mostly the MPI-centered research, more information for the AWI-centered research is here.
ONGOING RESEARCH THEMES (since 2016)
(c) R.L. Perez

(c) S. Rogge
(c) Y. Bodur
(c) *Will be updated soon*
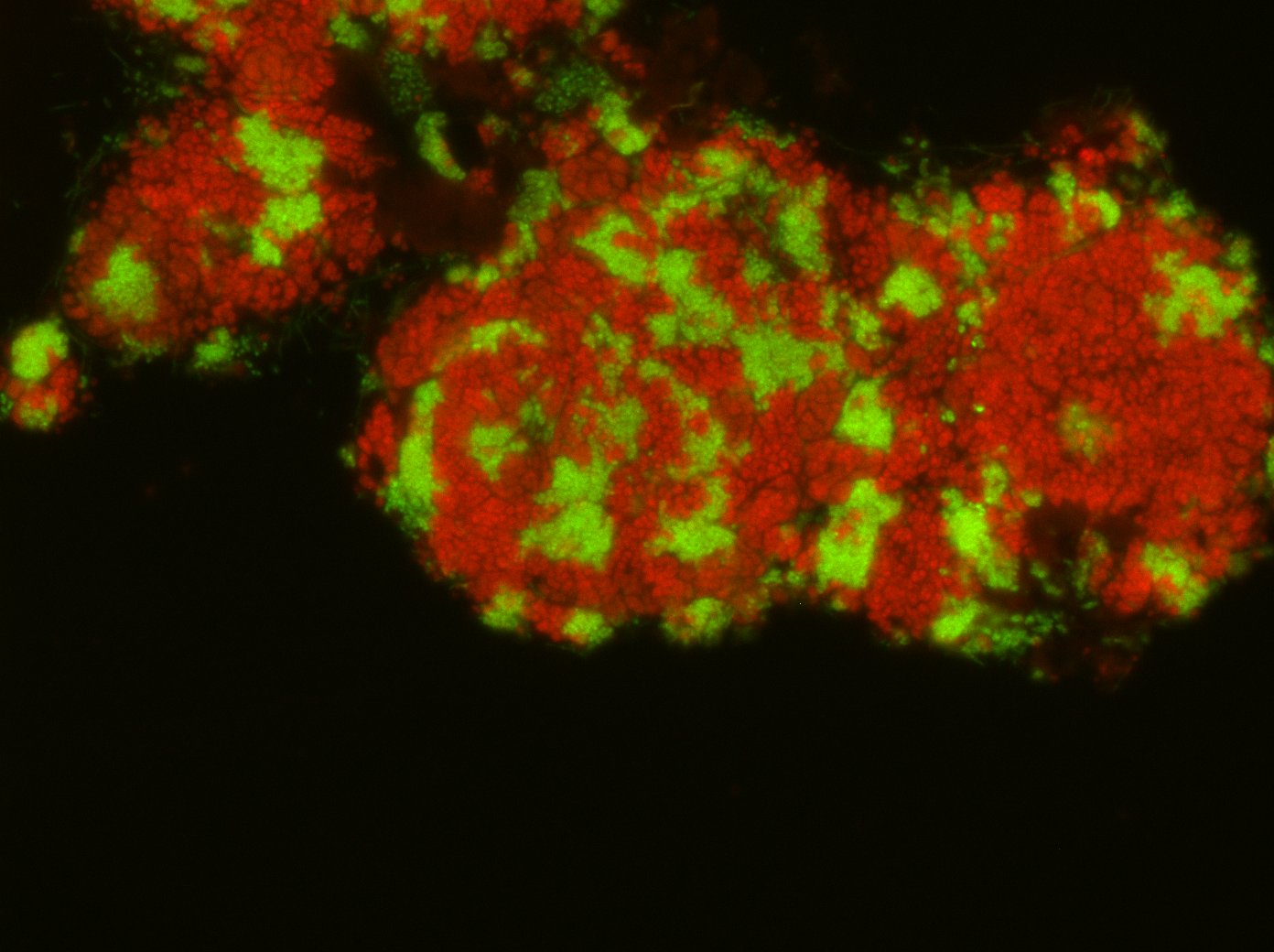
Microbial key players at seeps and vents
Research Project
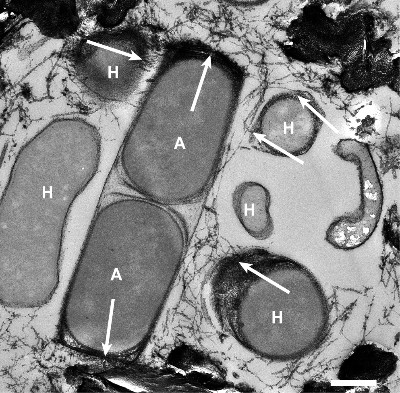
Seeps and vents haul reduced compounds, such as the hydrocarbons methane and ethane, as well as crude oils, hydrogen gas, and sulfide from the deep subsurface. Those compounds are potential energy sources for heterotrophic and chemoautotrophic microorganisms. Our team focuses on the anaerobic oxidation of alkanes by archaea in the seafloor. These archaea are abundant in gas-rich seep and vents and subsurface reservoirs and consume the greenhouse gas methane, ethane, but also long-chain alkanes. Additionally, we study the turnover of reduced compounds and microorganisms that thrive on these substrates at vent-derived hydrothermal plumes.
As basis of our research we explore and sample low temperature hydrocarbon seeps such as the Campeche Knolls in the Gulf of Mexico, the alkane-rich hydrothermal Guaymas Basin vents of the Gulf of California, and the hydrothermal vents of the Arctic spreading ridges. We apply a variety of in situ technologies such as benthic chambers, multisensor modules, and camera platforms, operated from remotely operated vehicles such as ROV Quest (MARUM, Bremen) and Ocean Floor Observation System (OFOS; AWI, Bremerhaven). On the ship we perform incubation experiments to assess turnover rates. In the home laboratories we use sediment samples from our expeditions to culture specific microorganisms that thrive on natural gas and oil constituents. We study the cultured organisms with molecular approaches, including sequencing techniques such as metagenomics and metatranscriptomics as well as metabolite analysis and fluorescence in situ hybridization.
Recent highlights include the cultivation of novel thermophilic archaea that at oxidize methane but also multi-carbon alkanes. We investigate their functioning and the catalysis in their key enzymes. We determine the imprint of microorganisms on methane-derived carbon, hydrogen, and clumped isotope patterns and the mechanisms underlying these pattern. This allows us a deeper understanding of subsurface carbon fluxes within the sediments. Water column projects include the microbiological characterization of hydrothermal vent and plume communities. By contribution to the Excellence Cluster ‘The Oceanfloor – Earth’s uncharted interface’ at MARUM, University Bremen we aim to integrate our results into regional and global carbon cycle models.
Contributors
Scientists & PhDs: Gunter Wegener (PI: lead), David Benito Merino, Cedric Hahn, Rafael Laso Pérez, Massimiliano Molari, Hanna Zehnle, Antje Boetius
Laboratory support: Susanne Menger, Martina Alisch
Financial support: DFG Cluster of Excellence ‘The Ocean Floor – Earth’s Uncharted Interface’ at MARUM, University Bremen
Former members: Viola Krukenberg, Emil Ruff
Key publications
- Ono, S., Rhim, J.H., Gruen, D.S., Tauber, H., Kölling, M., Wegener, G. (2021) Clumped isotopologue fractionation by microbial cultures performing the anaerobic oxidation of methane, Geochimica et Cosmochimica Acta 293, 70-85.
- Wang, Y., Wegener, G., Ruff, S.E., Wang, F. (2020) Methyl/alkyl‐coenzyme M reductase‐based anaerobic alkane oxidation in archaea. Environmental Microbiology.
- Hahn, C.J., Laso-Pérez, R., Vulcano, F., Vaziourakis, K.-M., Stokke, R. Steen, I.H., Teske, A., Boetius, A.. Liebeke, M., Amann, R., Knittel, K., Wegener, G. (2020) “Candidatus Ethanoperedens,” a Thermophilic Genus of Archaea Mediating the Anaerobic Oxidation of Ethane. mBio 11(2).
- Laso-Pérez R., Hahn C., van Vliet D.M., Tegetmeyer H., Schubotz F., Smit N.T., Pape T., Sahling H., Bohrmann G., Boetius A., Knittel K., Wegener G. (2019) Anaerobic degradation of non-methane alkanes by "Candidatus Methanoliparia" in hydrocarbon seeps of the Gulf of Mexico. mBio 10(4):e01814-19. doi:10.1128/mBio.01814-19
- Wang Y., Wegener G., Hou J., Wang F., Xiao X. (2019) Expanding anaerobic alkane metabolism in the domain of Archaea. Nature Microbiology. 4:595-602. doi:10.1038/s41564-019-0364-2r
- Krukenberg V., Riedel D., Gruber-Vodicka H. R., Buttigieg P.L., Tegetmeyer H.E., Boetius A. , Wegener G. (2018) Gene expression and ultrastructure of meso- and thermophilic methanotrophic consortia. Environ. Microbiol., 20(5):1651-1666 doi:10.1111/1462-2920.14077
- Laso-Pérez R., Krukenberg V., Musat F., Wegener G. (2018) Establishing anaerobic hydrocarbon-degrading enrichment cultures under strictly anoxic conditions. Nature Protocols, 13(6):1310-1330. doi:10.1038/nprot.2018.030
- Kellermann M.Y., Yoshinaga M.Y., Wegener G., Krukenberg V., Hinrichs K.-U. (2016).Tracing the production and fate of individual archaeal intact polar lipids by stable isotope probing, Organic Geochemistry. doi:10.1016/j.orggeochem.2016.02.004.
- Krukenberg V., Harding K., Richter M., Glöckner F.-O., Gruber Vodicka H., Adam B., Berg J., Knittel K., Tegetmeyer H.E., Boetius A., Wegener G. (2016) Candidatus Desulfofervidus auxilii, a hydrogenotrophic sulfate-reducing bacterium involved in the thermophilic anaerobic oxidation of methane. Environmental Microbiology, 18:3073-3091. doi: 10.1111/1462-2920.13283.
- Laso-Pérez R., Wegener G., Knittel K., Widdel F., Harding K. J., Krukenberg V., Meier D. V., Richter M., Tegetmeyer H. E., Riedel D., Richnow H.-H., Adrian L., Thorsten R., Lechtenfeld O., Musat F. (2016) Thermophilic archaea activate butane via alkyl-CoM formation. Nature. doi:10.1038/nature20152.
- Wegener G., Kellermann, M.Y., Elvert, M. (2016) Tracking activity and function of microorganisms by stable isotope probing of membrane lipids (invited review). Current Opinion in Biotechnology. doi: 10.1016/j.copbio.2016.04.022
- Wegener G., Krukenberg V., Ruff S.E., Kellermann M.Y., Knittel K. (2016). Metabolic capabilities of microorganisms involved in and associated with the anaerobic oxidation of methane. Frontiers in Microbiology. doi: 10.3389/fmicb.2016.00046.
- Yoshinaga, M.Y., Holler, T., Goldhammer, T., Wegener, G., Pohlman, J.W., Brunner, B., Kuypers, M.M.M., Hinrichs, K.-U., Elvert, M. (2014) Carbon isotope equilibration during sulphate-limited anaerobic oxidation of methane, Nature Geoscience. doi: 10.1038/ngeo2069
- Wegener, G., Bausch, M., Holler, T., Nguyen, M. T., Prieto Mollar, X.,, Kellermann, M.Y., Hinrichs, K-U. and Boetius, A. (2012) Assessing sub-seafloor microbial activity by combined stable isotope probing with deuterated water and 13C-bicarbonate. Microbiol. 14(6) 1517-1527 doi: 10.1111/j.1462-2920.2012.02739.x
- Kellermann, M.Y., Wegener, G., Elvert, M., Yoshinaga, M.Y., Lin, Y.-L., Holler, T., Prieto Mollar, X., Knittel, K., Hinrichs, K.-U. (2012) Autotrophy as a predominant mode of carbon fixation in anaerobic methane-oxidizing microbial communities, Proc Natl Acad Sc USA, 109 (49) 19321-19326. org/10.1073/pnas.1208795109
- Holler, T., Widdel, F., Knittel, K., Amann, R., Kellermann, M. Y., Hinrichs, K. U., Teske, A., Boetius, A. & Wegener, G. (2011). Thermophilic anaerobic oxidation of methane by marine microbial consortia. ISME J, 5(12), 1946-1956. doi: 10.1038/ismej.2011.77
- Wegener, G., Niemann, H., Elvert, M., Hinrichs K.-U. and Boetius, A. (2008) Assimilation of methane and inorganic carbon by microbial communities mediating the anaerobic oxidation of methane. Environ. Microbiol. 10 (9), 2287–2298. doi: 10.1111/j.1462-2920.2008.01653.x.
A complete record of publications can be found here
(c) R. L. Perez
Microbial community dynamics in the Arctic Ocean
Research Projects
FRAM (FRontiers in Arctic Marine Monitoring)
FRAM is a long-term observatory for year-round biological, oceanographic and biogeochemical observations in Fram Strait, the major gateway between the Atlantic and Arctic Ocean. FRAM builds on the Long Term Ecological Research Site (LTER) HAUSGARTEN established in 1999. The multidisciplinary approach of FRAM elucidates biological responses to fluctuations in ocean circulation, water mass properties and sea ice extent from surface to the seafloor, distinguishing natural variability from human impact. These insights contribute to understanding the effects of climate change on the Arctic Ocean, a region severely affected by rising water temperatures and Atlantification. Within the FRAM “Microbial Observatory”, our group studies the structure and function of microbial communities across Fram Strait, with emphasis on biogeographic and temporal variability as well as the connectivity between water, sea ice and sediment.
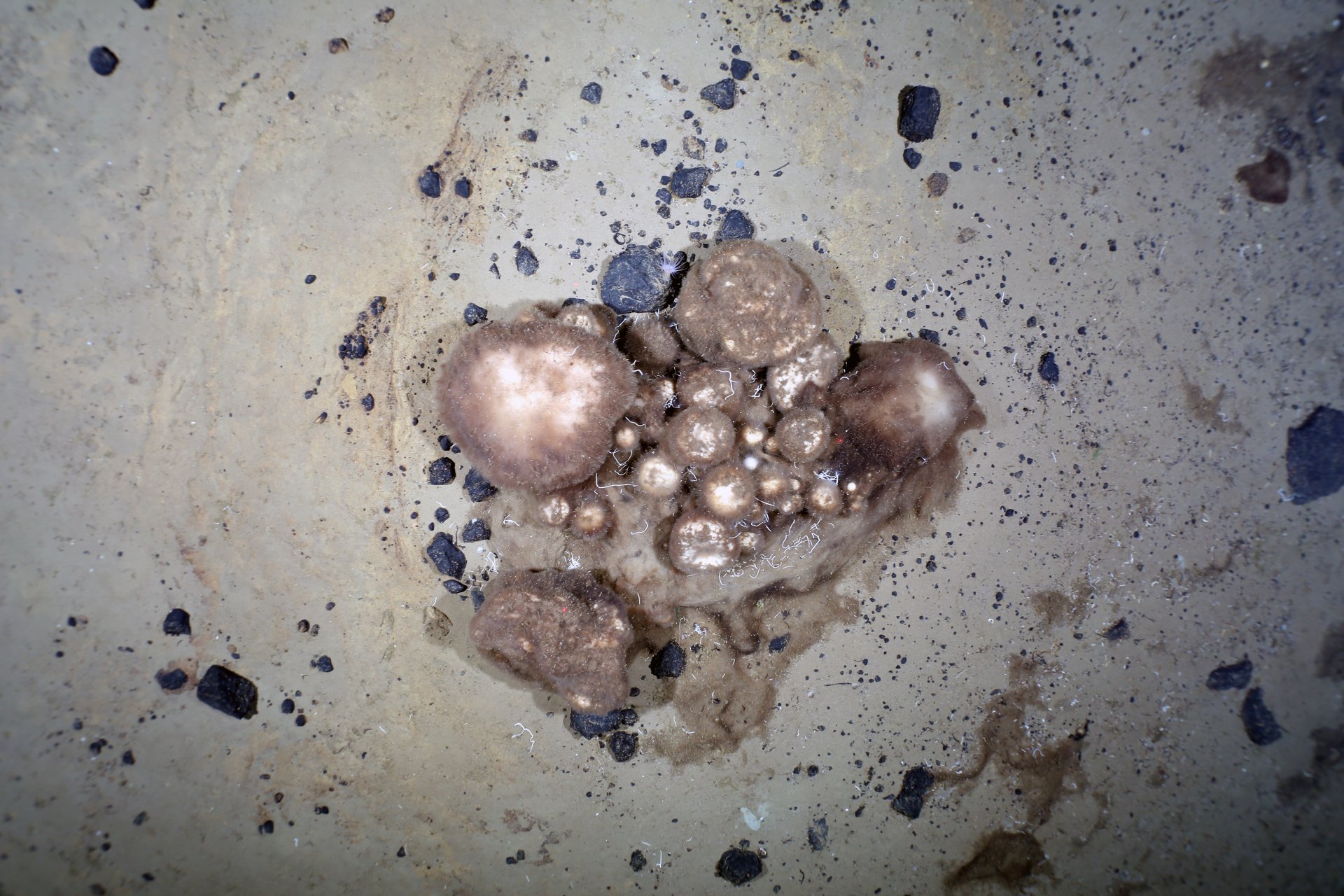
Feeding ecology of benthic communities in Central Arctic
With the aim to explore diverse ecosystems in Arctic Ocean, as an additional side project, our group explores the feeding ecology of benthic communities, mostly composed by bacteriosponges, dwelling on top of Karasik Seamounts in Central Arctic. The main objective is to elucidate the mechanisms that facilitate the presence and stability of dense sponge assemblage in oligotrophic conditions, with special emphasis on the role that microbial symbionts plays in the functioning of the holobiont. We use a multidisciplinary approach, including stable and compound specific isotope analysis, meta-genomics and meta-transcriptomics.
Objectives and methodologies
As part of the Microbial Observatory, we want to assess the impact of environmental changes on Arctic microbial biodiversity, function and distribution, decipher microbial interactions, and generate a better understanding of microbial processes that govern carbon flux and biogeochemical processes. Furthermore, we aim at establishing best practices for microbial observations in the Arctic Ocean .
- Spatial and long-term dynamics: annual sampling of pelagic and benthic communities from different water depths and water mass regimes across Fram Strait; analyzed by amplicon sequencing and metagenomics
- Seasonal variability: automated sampling of free-living and particle-associated microbial communities by autonomous devices (RAS water samplers and sediment traps) deployed on seafloor moorings; analyzed by amplicon sequencing
- Functional capacities and gene expression; analyzed by meta-omic sequencing
- Microbial communities in sea ice; connectivity between sea ice, water and sediment
- Feeding and functional ecology of bacteriosponge community and the role of associated microbes
- Ongoing collaborations with Katja Metfies, Eva-Maria Nöthig, Sinhue Torres-Valdes, Wilken-Jon von Appen and Morten Iversen (AWI) to contextualize prokaryotic information with phytoplankton growth patterns, nutrient budgets, oceanography and particle flux.
- In addition collaborations with Anna de Kluijver and Jack Middelburg (Utrecht Univ.) to analyze the stable and compound specific isotopes in sponges, and Beate Slaby and Ute Hentschel (GEOMAR) for meta-omic analysis. With AWI MICADAS Lab (https://www.awi.de/en/science/geosciences/marine-geochemistry/micadas.html) for radiocarbon measurements.
Recent cruises
- RV Polarstern PS124; Antarctic (2021)
- RV Polarstern PS121; Fram Strait (2019)
- RV Polarstern PS114; Fram Strait (2018)
- RV Maria S Merian MSM77; Fram Strait (2018)
- RV Polarstern PS109; Greenland (2017)
- RV Polarstern PS107; Fram Strait (2017)
- RV Polastern PS101 Karasik Seamounts (2016)
Contributors
Scientists and PhDs: Christina Bienhold, Matthias Wietz, Pier L. Buttigieg, Teresa Morganti, Magda Cardozo Mino, Felix Janssen, Frank Wenzhöfer, Antje Boetius
Laboratory Support: Jakob Barz, Susanne Menger, Martina Alisch, Theresa Hargesheimer (AWI)
Technical Support: Volker Asendorf, Axel Nordhausen, Elena Schiller (AWI)
Link:
https://www.awi.de/en/expedition/observatories/ocean-fram.html
Major achievements since 2017
- Identification of key pelagic and benthic bacterial groups in Fram Strait, as well as physical and biological processes driving spatial community dynamics (Cardozo Mino et al. Preprint, Fadeev et al. 2018, Hoffmann et al. 2017)
- Identification of sea ice and ice-algal aggregates as major drivers of carbon export and vertical microbial connectivity in the Arctic Ocean (Rapp et al. 2018, Fadeev et al. Preprint)
- First insights into seasonal and inter-annual dynamics and drivers of microbial community structure and function in Fram Strait (Wietz, Cardozo-Mino, Bienhold)
- Central Arctic seamounts host large biomass of Geodia sponges, which act as ecosystem engineers shaping benthic microbial communities (Morganti, Molari)
- Characterization of the phylogeny, environmental distribution, abundance and metabolic potential of Woeseiales bacteria, global members of marine sediment communities (Hoffmann et al. 2020)







(c) PS101 AWI OFOS system
(c) PS101 AWI OFOS system
Environmental impacts and risks of deep-sea mining
Research Project
The JPIO Project Mining Impact II is about the Environmental impacts and risks of deep-sea mining for Manganese (better: Polymetallic) Nodules. MiningImpact II involves institutions from all around Europe. While being integrated on the minister level, funding for the individual partners is provided by national funding agencies (BMBF in case of MPI) and runs from Aug. 2018 until end of Feb. 2022.
We are looking at disturbance effects on megafauna communities as well as microbial communities and their biogeochemical functions. In case of megafauna, investigations are mainly based on high-resolution imaging and video observations of the seafloor by means of OFOS surveys. Methods to investigate microbial communities will include NGS studies of DNA and to some extent RNA, microscopy (AODC, (CARD)FISH). Assessment of effects on biogeochemical functions will be based on DIC & extracellular enzymatic activities as well as on respiration rates calculated from in situ microsensor profiles and in situ nodule incubations.
Recent Cruises
SO268/1 (PI: P. Linke, GEOMAR): 17. Feb. 2019 - 29. Mar. 2019 (Manzanillo, MEX - Manzanillo, MEX)
SO268/2 (PI: M. Haeckel, GEOMAR): 1. Apr. 2019 - 28. May. 2019 (Manzanillo, MEX - Manzanillo, MEX)
MiningImpactII cruise - Mangan21 (PI: Annemiek/BGR) Island Pride provided by Ocean Infinity and chartered by BGR: 4. Apr. 2021 to mid May 2021 (about 42 days) (San Diego - San Diego, USA)
Working areas are in the German and Belgium License Area in the eastern Part of the Clarion Clipperton Fracture Zone (CCZ) in the tropical NE Pacific.
For further information please follow: https://miningimpact.geomar.de/
Contributors
Scientists & PhDs: Felix Janssen (PI: Project Management), Frank Wenzhöfer (PI: In situ respiration studies), Massi Molari (PI: Microbial Communities & Functions), Autun Purser (PI: Megafauna & Habitat; AWI), Julia M. Otte (Microbial Communities), Batuhan C. Yapan (Microbial Communities), *Yasemin Bodur (Megafauna), Tanja Stratmann, Antje Boetius
(*former members of the group)
Laboratory Support: Jakob Barz, Susanne Menger
Technical Support: Volker Asendorf, Axel Nordhausen, Elena Schiller (AWI)
Links
SO268 Cruise Blog:
https://www.oceanblogs.org/eadsm/
Movie about our work on board:
https://www.youtube.com/watch?v=YiJOUBdi4J0&feature=youtu.be
Television Report about our cruise:
https://www.zdf.de/nachrichten/zdf-morgenmagazin/moma-future-bergbau-am-meeresgrund-100.html
Major achievements since 2019
- Molari, M., Janssen, F., *Vonnahme, T. R., Wenzhöfer, F. and Boetius, A. (2020): The contribution of microbial communities in polymetallic nodules to the diversity of the deep-sea microbiome of the Peru Basin (4130-4198 m depth) , Biogeosciences, 17, 3203-3222. doi: 10.5194/bg-17-3203-2020
- *Vonnahme T.R., Molari M., Janssen F., Wenzhöfer F., Haeckel M., Titschack T., Boetius A. Effects of a deep-sea mining experiment on seafloor microbial communities and functions after 26 years. Science Advances. Science Advances. 29 Apr 2020: Vol. 6, no. 18, eaaz5922. DOI: 10.1126/sciadv.aaz5922.


(c) J.Otte

(c) J.Otte

(c) J.Otte
(c) Y. Bodur
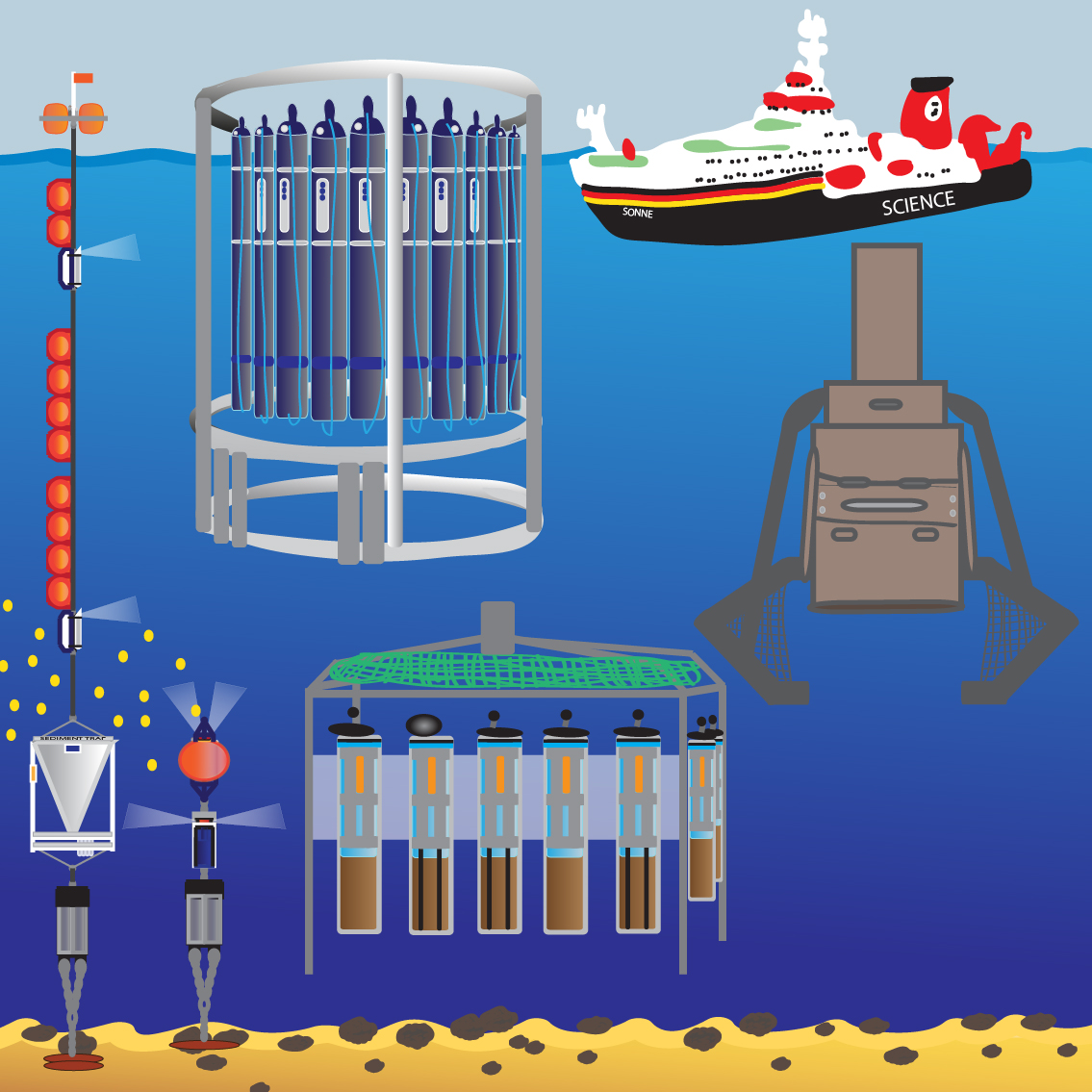
In situ and in silico technologies for studies of microbial communities and functions
Research Projects
In situ technologies
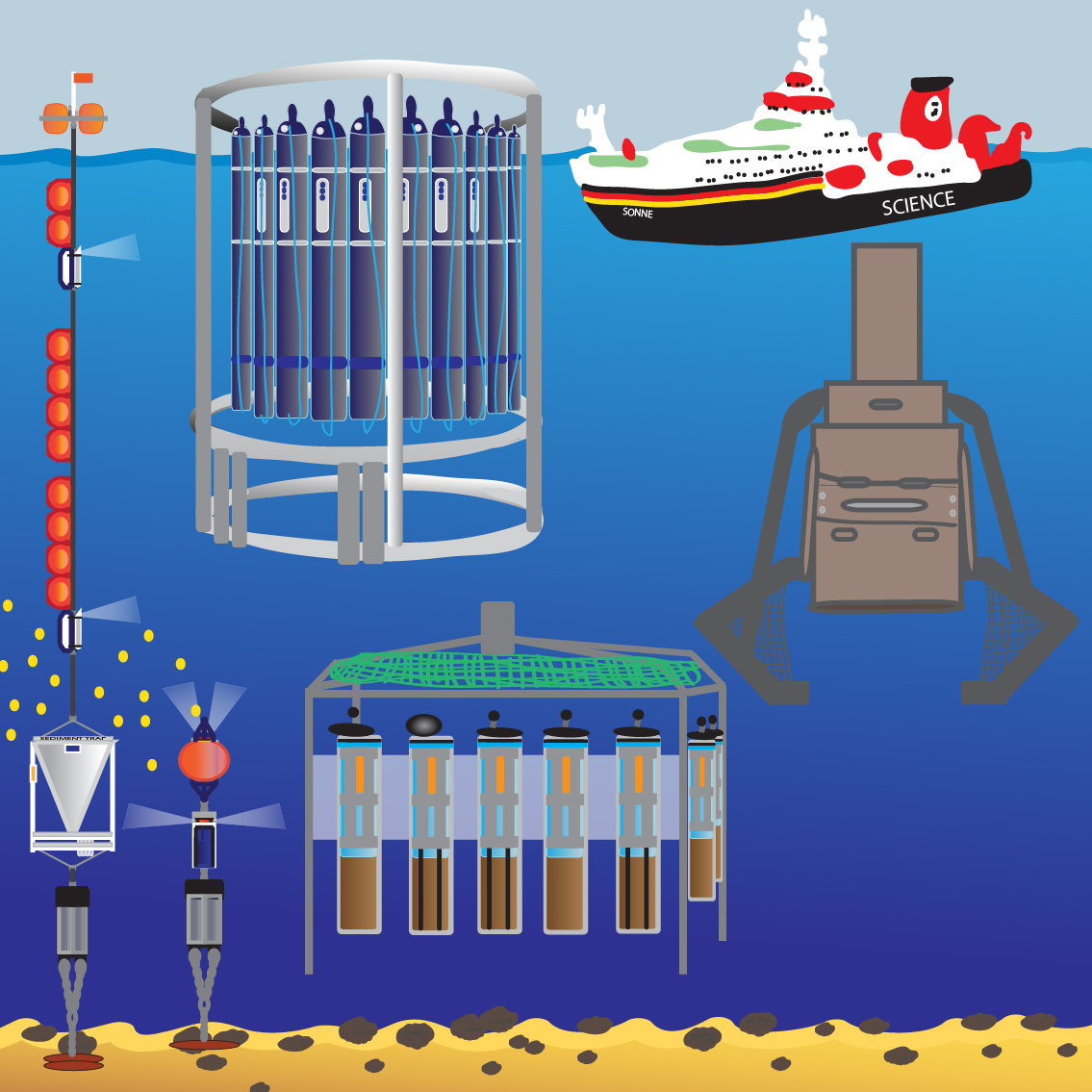
Remote polar and deep-sea regions host many unexplored ecosystems with unknown functions in biogeochemical cycles and unexplored biodiversity. Because of technological and logistical challenges, in situ observations of environmental processes and habitat dynamics are still challenging and only exist in very limited numbers and spatial coverage. Innovative robotic technologies have become key to study processes and changes in space and time. Within the reporting period, we have substantially improved the use of sensor systems and underwater robotic platforms for deep-sea research, biogeochemistry and microbial ecology. These include small systems manipulated by ROVs, as well as fully integrated autonomous benthic crawler and free-falling lander systems. During expeditions to extreme environments such as hadal trenches and ice-covered polar regions, we have been able to combine a variety of new technologies to investigate previously uncharacterized environments and microbial habitats.
In silico technologies
The remoteness of the polar and deep-sea ecosystems studied by the DSET group also has consequences for the handling and dissemination of the data, information, and knowledge we generate. The rarity of these outputs (relative to studies of, e.g., the coasts and surface waters) can reduce their visibility in the global data ecosystem. To address this, members of the group are active in international consortia developing “in silico” technologies to better expose new knowledge of the polar, deep-sea regions to both human and machine agents. In particular, members of our group are leaders in the field of environmental Knowledge Representation (KR). KR is a branch of Artificial Intelligence (AI) which studies the nature of meaning (i.e. semantics) and translates human knowledge into machine readable forms, captured in digital, web-accessible artifacts known as “ontologies”. Through leading roles in the Earth Science Information Partners (ESIP) and the Open Biological and Biomedical Ontologies Foundry, we transfer new knowledge from our research themes into reference digital technologies the global data ecosystem, while simultaneously studying the digitisation of knowledge itself. Further and during the reporting period, we have been increasingly active in high-level global efforts to evolve the current ocean data system, especially through activities in IOC-UNESCO working groups and through active contributions to the “Transparent and Accessible Ocean” theme during the preparatory process UN Decade of Ocean Science for Sustainable Development.
Contributors
Scientists: Frank Wenzhöfer, Pier Luigi Buttigieg, Felix Janssen.
Technical Support: Axel Nordhausen, Volker Asendorf, Fabian Schramm
Major achievements since 2017
- Successful implementation of an in situ experiment to show benthic community response to changing phytodetritus input to the Arctic deepsea (Braeckman et al. 2018)
- Revealing patterns of Arctic deep-sea communities controlled by independent abiotic environmental factors (ice-cover, food availability, water depth) (Hoffmann et al., 2018)
- In situ studies of ice algal production and its importance under various ice conditions in the Arctic (Attard et al., 2018)
- Gaining new knowledge on trench ecosystems and deep sea ecosystems in general using a multidisciplinary, concerted and quantitative approach in comparing carbon and nutrient fluxes as well as the structure, composition, and connection of hadal communities (RV SONNE cruise SO261 Atacama Trench & TAN1711 Kermadec Trench)
- Development and application of an autonomous lander system for in situ sediment incubation (tracer injection) and core recovery from hadal depths
- Operation of 2 autonomous benthic crawler systems for longterm-term (1-year missions) to investigate seasonal variations in benthic community respiration in Arctic deep-sea sediments since 3 years (PS108, MSM77, PS121)
- Integration of semantic technologies with polar and deep-sea content into international data architectures including
- IOC-UNESCO Ocean Data and Information System (ODIS)
- The Earth Science Information Partners (ESIP) Semantic Web for Earth and Environmental Technology (SWEET)
- Contributions to international working groups for Polar Semantics, in conjunction with collaborators including the World Meteorological Organization (WMO) and the US National Snow and Ice Data Center (NSIDC)
- Participation in the IOC-UNESCO IODE Inter-sessional working group to propose a strategy on ocean data and information stewardship for the UN Ocean Decade of Ocean Science for Sustainable Development (IWG-SODIS)
- Initialisation of an EU COST action - CAPARDUS - to build an Arctic Best Practices system to harvest, preserve, and interlink knowledge in the region, including indigenous knowledge
- Leading the technical components and strategy of the recently-launched IOC-UNESCO Ocean Best Practices System (Buttigieg et al., 2019)
- Contributions to the data science infrastructure supporting the Group on Earth Observation Biodiversity Observation Networks (GEO BON) Essential Biodiversity Variables (EBVs) and the Global Ocean Observing System (GOOS) Essential Ocean Variables (EOVs)
- Founding the Global Omics Observatory Network and building new data exchange and standardisation mechanisms to further molecular observation of life in the ocean
PREVIOUS RESEARCH THEMES (until 2016)
Functional diversity of marine microbiomes
Scientists: Antje Boetius (main PI), Christina Bienhold, Pier Luigi Buttigieg, Verena Carvalho, Eduard Fadeev, *Mar Fernandez-Mendez, Cedric Hahn, *Christiane Hassenrück, *Katy Hoffmann, Marianne Jacob, *Gerdhard Jessen, *Viola Krukenberg, Rafael Laso-Pérez, Massimiliano Molari, *Pierre Offre, Claudia Pala, *Alban Ramette, Josephine Rapp, Pamela Rossel, *Emil Ruff, Halina Tegetmeyer, *Tobias Vonnahme, Gunter Wegener; Laboratory support: Jakob Barz, Susanne Menger, Wiebke Stiens, Erika Weiz-Bersch; Technical Support: Volker Asendorf, Axel Nordhausen, Fabian Schramm
*former members of the group
Describing microbial diversity and its drivers in complex natural environments represents a cornerstone of modern microbial ecology. Similar questions and approaches drive this research in different fields ranging from extreme deep-sea habitats to the human microbiome. The technological revolution of the field by fast new sequencing techniques has also transformed concepts of microbial diversity and community turnover at different spatial and temporal scales. We develop data pipelines and expert knowledge systems to provide a statistical resource to analyze datasets of increasing complexity and size. The aim is to resolve the identity and metabolism of taxa defining the core microbiome of marine environments. We investigate microbial life in polar environments, deep-sea floor and trenches, anoxic basins, mud volcanoes, gas and oil seeps, hydrothermal vents, food falls, manganese nodules, and coral reefs. These habitats are characterized by different biological, geological, and physical conditions, showing substantial temporal and spatial variations. Combining ‘omics approaches with experimental and field work enables the characterization of the ecological roles of several uncultivated microbial groups as well as the interplay between microorganisms and their energy sources.
Main recent achievements include:
- Bienhold et al. (2016) identified core bacterial taxa in a global survey of marine seafloor environments
- Ruff et al. (2015) identified core taxa and key members of functional groups in cold seep environments
- Rossel et al. (2016) performed high-resolution molecular profiles of dissolved organic matter (DOM) from sediment porewaters of the deep Eurasian basins in relation to environmental parameters
- Hoffmann et al. (2017) identified patterns in deep-sea microbial communities in response to natural nutrient inputs
- Fernández-Méndez et al. (2016) identified microbial nitrogen fixation potential in the nitrogen-limited Central Arctic Ocean
- Schöttner et al. (2013) identified the complex host-microbe diversity and co-evolutionary patterns in cold water coral reef sponges
- Furthermore, we continuously test and re-evaluate the in-depth analyses of classical vs. NGS tools to explore microbial diversity in natural environments. Different generations of molecular techniques may be complementarily used to meaningfully describe microbial diversity and its drivers
- Also, we permanently optimize our statistical toolbox (interactive guide and software package) to obtain more transparent and reproducible analytical procedures in our field
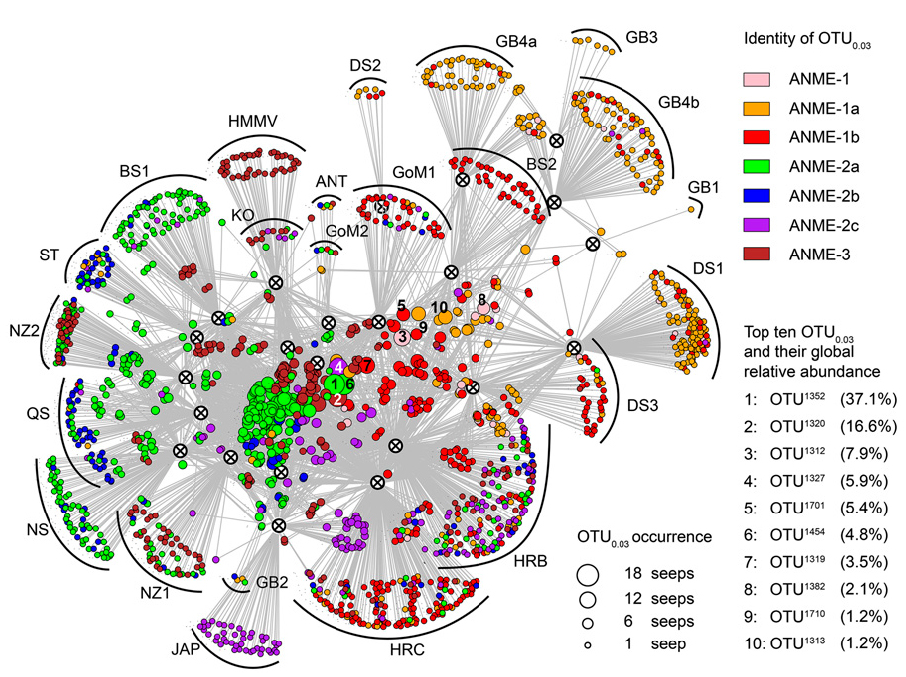
Geosphere-biosphere interactions and anaerobic hydrocarbon degradation in extreme environments
Scientists: Gunter Wegener (main PI), Antje Boetius, *Viola Krukenberg, Rafael Laso-Pérez, Massimiliano Molari, Pamela Rossel, *Emil Ruff, *Alban Ramette, *Tobias Vonnahme, Frank Wenzhöfer; Laboratory support: Jakob Barz, Mirja Meiners, Susanne Menger; Technical support: Fabian Schramm, Axel Nordhausen
* former members of the group
Extreme environments are defined by one or more physicochemical parameters, such as e.g. extremely high or low temperature, salinity, pH, and energy availability, at which life operates close to its known limits. Cold seeps and hot vents habitats represent extreme environment at which cold or hot fluids from the subsurface are emitted to the seafloor that are enriched in reduced compounds such as methane, short- and long-chain hydrocarbons, and hydrogen. We aim at a functional understanding on the variety the microorganisms that harvest the energy from these fluids and are the basis of completely light-independent ecosystems in the deep oceans. Therefore we visit hot vents in the Gulf of Mexico (Campeche Hydrocarbon field), the Gulf of California (Guaymas Basin), and the Arctic Ocean (Gakkel Ridge). Other types of extreme environments studied by international collaborations are deep-sea trenches, mud volcanoes, and CO2 vents. Currently we study the distribution and genomes of microorganisms in the plumes of vents and in hydrocarbon-rich sediments. Therefore, we use a variety of in situ technologies such as benthic chambers, multisensory modules, and camera platforms, operated from remotely operated vehicles such as ROV Quest (MARUM, Bremen), or as autonomous lander systems. In the home laboratories we cultivate specific microbial groups thriving on energy-rich components in the geofluids, such as methane, short-chain hydrocarbons, and hydrogen, and study their physiology in experiments by using a variety of molecular approaches, including metagenomics, metatranscriptomics, and in situ hybridization.
Main recent achievements include:
- Wegener et al. (2015) identified nanowires and cytochromes that enable direct electron transfer from the ANME to their partner bacteria
- Krukenberg et al. (2016) isolated HotSeep-1, the partner bacterium in thermophilic AOM and other hydrocarbon degrading consortia
- Laso-Perez et al. (2016) identified the process of butane and propane activation via alkyl-CoM formation, analogous to the oxidation of methane, in some archaeal-bacterial consortia
- Pop-Ristova et al. (2015) investigated the development of wood falls into chemosynthetic habitats
- Boetius and Wenzhöfer (2013) quantified the efficiency of the benthic filter for methane at continental margins worldwide
- Furthermore, we continuously study how in situ temperature variations shape the microbial diversity in the Guaymas Basin sediments
- Also, we permanently develop novel stable isotope probing-based approaches to quantify microbial productivity, carbon fixation, and transformation under extreme environmental conditions
Global change effects on microbial communities and functions
Scientists: Christina Bienhold (main PI), Antje Boetius, *Ulrike Braeckmann, Pier Luigi Buttigieg, *Christiane Hassenrück, *Katy Hoffmann, Marianne Jacob, Felix Janssen, *Gerdhard Jessen, *Mar Fernández-Méndez, Massimiliano Molari, *Pierre Offre, Josephine Rapp, *Alban Ramette, *Tobias Vonnahme, Frank Wenzhöfer; Laboratory support: Martina Alisch, Jana Bäger, Jakob Barz, Rafael Stiens, Wiebke Stiens, Erika Weiz-Bersch; Technical support: Axel Nordhausen, Volker Asendorf
* former members of the group
Oceanic ecosystems experience various environmental pressures, many of which are a consequence of human activities, such as anthropogenic carbon dioxide emissions, loss of sea ice by warming, pollution by hydrocarbons, and declining oxygen concentrations by eutrophication. Effects of such activities can already be recognized in remote polar and deep-sea environments. Our work aims at studying and quantifying the role of microorganisms in global change effects and feedback mechanisms to better understand future changes in marine ecosystems, which is one of the aspects of the ERC AdvG project Abyss. Furthermore, we contribute to the establishment of baselines for ecosystem status, which are largely missing in remote ocean ecosystems. In addition to fieldwork and laboratory studies, we carry out long-term observations, especially at the LTER site HAUSGARTEN in Fram Strait, which is one of the few biogeochemical deep-sea observatories on Earth.
Main recent achievements include:
- Boetius et al. (2013) describe massive export of algal biomass from the sea ice to the seafloor as a result of sudden warming, causing fast reactions in the entire ecosystem from ocean productivity to export fluxes to the deep sea
- Boetius et al. (2015) review current knowledge about bacterial diversity across sea ice, pelagic, and benthic ecosystems in the central Arctic Eurasian basin and present fundamental links between the dynamics in microbiomes and the rapidly changing cryosphere
- Jacob et al. (2013), Soltwedel et al. (2015) and Buttigieg and Ramette (2015) revealed that interannual variations in ocean surface temperatures and sea ice cover st the LTER site HAUSGARTEN (Fram Strait) and in the Central Arctic are reflected in benthic community structure and changes in the deposition of organic matter
- Hassenrück et al. (2015, 2016) studied the effects of changing CO2 concentrations on benthic communities at natural CO2 vents, as analogues to ocean acidification, and showed that high CO2 levels cause diversity shifts, suggesting a coping mechanism for community resilience
- Purser et al. (2016) revealed the association of deepsea octopods breeding with manganese crusts and nodules in the Pacific Ocean
- Jessen et al. (2017) identified responses in microbial activity and community diversity to fluctuating hypoxia, and quantified the consequences for carbon burial
- Furthermore, we conduct continuous autonomous monitoring of oxygen dynamics at high spatial and temporal resolutions to better understand the effects of temporal hypoxia on ecosystems. We can show that short-term variations in oxygen fluxes have significant effects on benthic ecosystems, including differences in the preservation of organic matter, as well as the composition and diversity of bacterial communities.
- Also, we study the long-term effects of large-scale disturbances and re-colonization by an experimental setup (DISCOL) located in the South Pacific Ocean, simulating deep-sea mining on benthic communities. Initial observations suggest that mining can disturb seafloor communities for decades by disrupting the active surface layer.
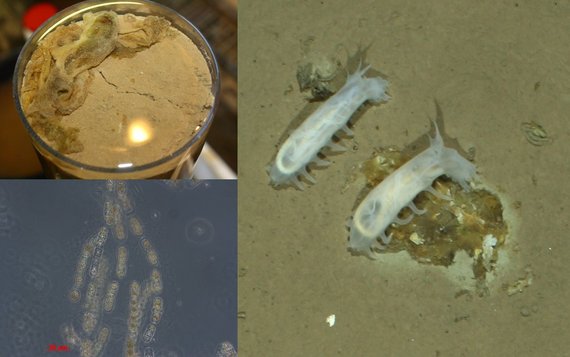
Method developments: In situ technologies for microbial habitat studies
Scientists: Frank Wenzhöfer (main PI), Antje Boetius, *Daphne Donis, *Janine Felden, Ralf Hoffmann, Felix Janssen; Lab support: Martina Alisch, Mirja Meiners, Rafael Stiens, Erika Weiz-Bersch; Technical support: Volker Asendorf, Michael Hofbauer, Karin Hohmann, Axel Nordhausen, Fabian Schramm, and the MPI workshops
*former members of the group
Investigations of deep-sea ecosystems and their organisms, as well as related biogeochemical processes rely on the constant development of technology that enables in situ analyses and observations directly at the seafloor. Many processes occur at temporal and spatial scales that cannot be effectively studied after sample retrieval due to depressurization and warming, or the mortality of deep-sea animals. Thus, assessments of biological, geological, physical, and chemical processes in deep waters, as well as long-term observations of deep-sea ecosystems, require the design and development of new technology, especially with regard to energy supply, sensor configuration, and data communication. Innovative robotic technologies have become key to study ocean processes and changes in space and time. We have substantially improved the use of chemical and biological sensor systems and underwater platforms for deep-sea research, biogeochemistry and microbial ecology. We can equip diverse underwater platforms (e.g. ROVs, AUVs, crawler, submersibles, autonomous lander, towed and moored systems) with sensors and cameras for coverage at a wide range of temporal and spatial scales. The technological developments enable us to study the deep seafloor of a variety of extreme habitats, like the Central Arctic, deep trenches, CO2 seeps and vents including under-ice ecosystems of polar regions. In the ice-covered Central Arctic we used newly developed moored benthic lander systems to quantify export of organic material to the deep seafloor, measuring the total benthic community respiration in situ by benthic chamber and microprofiler.
Furthermore, we assist in long-term strategies to investigate the seasonal variation at the deep-sea floor. For example, a long-term microprofiler has been deployed at HAUSGARTEN in 2013 for one year. The system consists of an optical oxygen sensor array, which runs vertical profiles across the sediment-water interface every week moving a swinging arm horizontally between each vertical profile during its 12-month deployment.
Main recent achievements with technical aspects include:
- Testing the sensitivity of eddy sensing systems for benthic flux measurements in the deep sea (Donis et al. 2015, 2016)
- Miniaturization of payload systems (sensor and incubation systems) for biogeochemical process studies operated by ROVs (Pop-Ristova et al. 2015, 2017)
- Operations of sea ice ROVs and tethered HROV in ice-covered environments - sea ice, vents, and seamounts in the Central Arctic (Katlein et al. 2015a, 2015b)
- Design of hadal sampling, incubation, and measuring lander-systems (Wenzhöfer et al. 2016)
- Development and deployments of a towed bathymetry camera system equipped with sonar and camera systems (OFOBS Ocean Floor Observation Bathymetry System), increasing surveyed regions from 3 m camera views to >30 m paths (Purser et al. 2016)
- Furthermore, we continuously develop integrated payload systems (multi-sensor and -sampling systems) for ROV, AUV, towed instrument (OFOS) and crawler operations (e.g. EU-Eurofleets), and validate sensor systems for measurements at extreme conditions and for long-term applications (e.g. EU ITN-SenseNet).
- Also, we continuously development under-ice deep-sea benthic lander system, construct instrument systems for biogeochemical studies in and directly under sea ice, design a combined chemical and optical observation system to explore deep-sea environments online, and optimize pipelines to annotate, archive and provide deep-sea photos and videos.